Ultrafast spectroscopy of solvated electrons in polar in ionic liquids
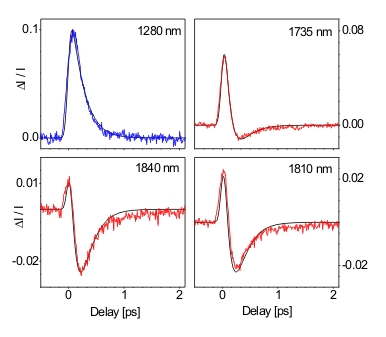
Understanding electron solvation is of fundamental interest in chemistry and crucial for a number or different phenomena such as chemical reduction of aromatic compounds by alkali metal-ammonia solutions, coexistence with anions in negatively charged clusters and interaction at interfaces (2-dimensional localization). Often, solvated electrons appear only for a short period of time as intermediates in complex environments, which makes it difficult to gain more information on the physical properties of these species. Therefore, easily accessible systems are desirable with well-defined initial parameters, e. g. concentration. Solvated electrons in ammonia – so-called ammoniated electrons – can be synthesized by dissolving alkali metal in liquid ammonia resulting in a clue colour at low concentrations becoming bronze at high concentration (non-metal/metal-transition).

In collaboration with the group of Prof. P Vöhringer we performed the first-ever femtosecond pump-probe experiments on ammoniated electrons and simulated in terms of an ultrafast temperature jump scenario (black lines).
Further reading (including results in water):
[1] A.-N. Unterreiner, „Ultraschnelle Relaxationsdynamik solvatisierter Elektronen in flüssigem Ammoniak und Wasser” (Ultrafast relaxation dynamics of solvated electrons in liquid ammonia and water)
Verlag Mainz, Aachen, 1998; zugl. Dissertation, Universität Karlsruhe
[2] A. Hertwig, H. Hippler, A.N. Unterreiner and P. Vöhringer, “Ultrafast Relaxation Dynamics of Solvated Electrons in Water”
Ber. Bunsenges. Phys. Chem., 102, 805-810 (1998)
[3] A. Hertwig, H. Hippler, and Andreas-N. Unterreiner, “Transient spectra, formation, and geminate recombination of solvated electrons in pure water UV-photolysis: an alternative view”
Phys. Chem. Chem. Phys., 1, 5633-5642 (1999)
[4] Andreas Hertwig, Horst Hippler and Andreas-N. Unterreiner, “Temperature-dependent studies of solvated electrons in liquid water with two and three femtosecond pulse sequences”
Phys. Chem. Chem. Phys., 4 (18), 4412-4419 (2002)
[5] Andreas-Neil Unterreiner, Jörg Lindner, and Peter Vöhringer, “Ultrafast Dynamics of solvated electrons in liquid ammonia solutions”
Femtochemistry and Femtobiology: Ultrafast Dynamics in Molecular Science (World Scientific Publishing House), Toledo, Spain, Sept. 2-6, 2001, 261-269
[6] Andreas-N. Unterreiner, Jörg Lindner, and Peter Vöhringer, “Ultrafast Relaxation Dynamics of the Solvated Electron in Liquid Ammonia”
in Ultrafast Phenomena XIII, Eds. R. D. Miller, M. M. Murnane, N. F. Scherer, A. M. Weiner, Springer-Verlag, 71, 468-470 (2003)
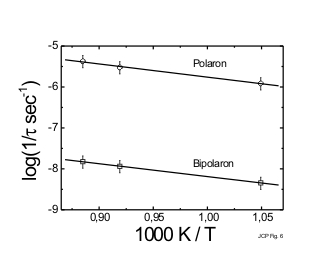
Ultrafast dynamics of excess electrons in Na-NaBr and Na-NaI molten solutions at elevated temperatures (T = 953 – 1128 K) were investigated with 80 fs time resolution. Polaron and bipolaron dynamics can be identified by a transient bleach at various probe wavelengths with time constants of t1 = (200 ± 35) fs and t2 = (2.8 ± 0.3) ps for Na-NaBr melt at 1073 K, and t1 = (370 ± 40) fs and t2 = (4.2 ± 0.3) ps for Na-NaI melt at 953 K. A transient absorption at longer wavelengths, which decays with the same time constants as the transient bleach recovery is attributed to Drude-type electrons. In agreement with our earlier results for K-KCl melts the fast component was attributed to the relaxation of Drude-type electrons into polarons. t2, on the other hand, represents the time during which Drude-type electrons recombine with polarons leading to bipolaron formation. The slower relaxation rates in Na-NaI melt can be attributed to the temperature dependence of the ionic diffusion coefficient. At higher temperatures where the ionic diffusion in Na-NaI melts becomes comparable to Na-NaBr melts, the time constants also coincide. For Na-NaI melts an activation energy of (25 ± 5) kJ/mol, was obtained from the temperature-dependent investigations of the relaxation dynamics. These investigations suggest that ionic diffusion facilitates the electron relaxation in alkali metal doped alkali halide melts.
Further reading:
[1] Stanislav Dogel, Werner Freyland, Horst Hippler, Detlef Nattland, Chandrasekhar Nese and Andreas-N. Unterreiner, “Ultrafast dynamics of excess electrons in a molten salt: Femtosecond investigation of K-KCl melts”
Phys. Chem. Chem. Phys., 5, 2934-2937 (2003)
[2] H. Brands, N. Chandrasekhar, H. Hippler, and A.-N. Unterreiner, “Femtosecond dynamics of excess electrons in a molten Na-NaBr system”
in Femtochemistry and Femtobiology, Eds. M. M. Martin and J. T. Hynes, Elsevier B. V., 249-253 (2004)
[3] H. Brands, H. Hippler, N. Chandrasekhar, and A.-N. Unterreiner, “Ultrafast dynamics of excess electrons in molten salts: II. Femtosecond investigations of Na-NaBr and Na-NaI melts"
submitted to J. Chem. Phys. (2005)
