Atmospheric Chemistry and Low-Temperature Combustion
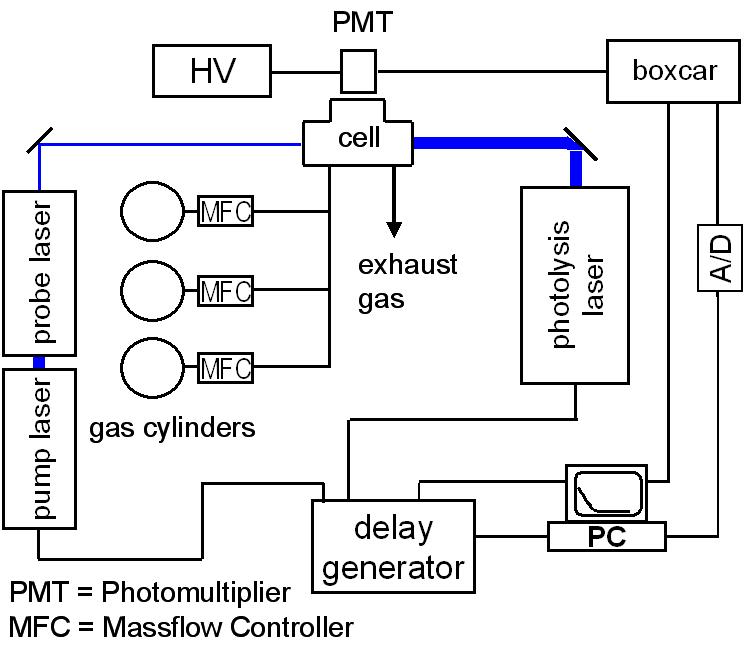
The temperature range characteristic for atmospheric chemistry and low-temperature combustion is covered by our laser photolysis setups and a recently established flow tube with mass-spectrometric detection.
In the laser-photolysis experiments, radicals are generated from suitable precursors by pulsed lasers. The reaction progress is monitored usually with laser-induced fluorescence. We use different experimental setups that allow covering a temperature range from 100 to 600 K and a pressure range from 10 mbar up to 200 bar. These techniques are used to determine rate coefficients and relative product yields over extended temperature and pressure ranges. The final goal is to obtain a detailed understanding of the elementary chemical reaction and to provide modelers with reliable kinetic and thermodynamic data.
Schematic picture of the experimental setup for laser-induced fluorescence
Because the OH radical is both a major cleaning agent in the troposphere and an important chain carrier in combustion, we extensively study reactions of OH radicals with organic species [1,2], including potential biofuels [3,4]. Sometimes our experiments are supplemented by mechanism development [5].
[1] C. Bänsch, J. Kiecherer, M. Szöri, M. Olzmann, The reaction of dimethyl ether with hydroxyl radicals: kinetic isotope effect and prereactive complex formation, J. Phys. Chem. A 117, 8343 (2013).
[2] A. J. C. Bunkan, J. Hetzler, T. Mikoviny, A. Wisthaler, C. J. Nielsen, M. Olzmann, The reactions of N-methylformamide and N,N-dimethylformamide with OH and their photo-oxidation under atmospheric conditions: experimental and theoretical studies, Phys. Chem. Chem. Phys. 17, 7046 (2015).
[3] C. Bänsch, M. Olzmann, Reaction of dimethoxymethane with hydroxyl radicals: An experimental kinetic study at temperatures above 296 K and pressures of 2, 5, and 10 bar, Chem. Phys. Lett. 720, 19 (2019).
[4] C. A. Whelan, J. Eble, Z. S. Mir, M. A. Blitz, P. W. Seakins, M. Olzmann, D. Stone, Kinetics of the reaction of hydroxyl radicals with furan and its alkylated derivatives 2-methyl furan and 2,5-dimethyl furan, J. Phys. Chem. A 124, 7416 (2020).
[5] J. Eble, J. Kiecherer, M. Olzmann, Low-temperature Autoignition of Diethyl Ether/O2 Mixtures: Mechanistic Considerations and Kinetic Modeling, Z. Phys. Chem. 231, 1603 (2017).