Combustion Chemistry and Shock Tube Experiments
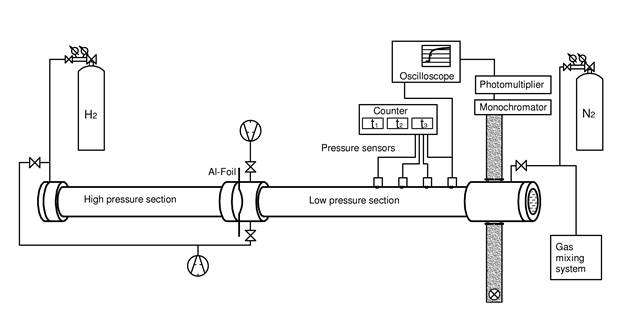
In shock-tube experiments, reactions are initiated by a temperature jump behind a shock wave. The shock wave is generated by pressure-induced bursting of an aluminum foil that initially separates the high- and low-pressure section of the shock tube. Temperatures between 800 and 3000 K are accessible with our equipment at pressures between 0.1 and 10 bar. We monitor the progress of reaction by either time-resolved absorption or time-resolved mass spectrometry.
Schematic picture of a shock tube
Most recent projects include pyrolysis and oxidation reactions of oxygenated species/biofuels [1-3] to determine or validate kinetic parameters for combustion modeling. We also studied high-temperature reactions of NCN [4] because they are of interest for understanding the formation of NOx in combustion under fuel-rich conditions.
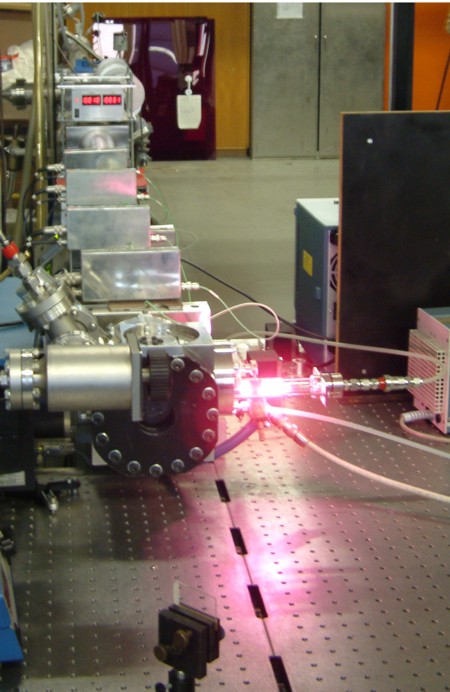
Head of the shock tube with microwave discharge lamp for atomic resonance absorption spectrometry
[1] J. Kiecherer, C. Bänsch, T. Bentz, M. Olzmann, Pyrolysis of ethanol: a shock-tube/TOF-MS and modeling study, Proc. Combust. Inst. 35, 465 (2015).
[2] L. Weiser, I. Weber, M. Olzmann, Pyrolysis of furan and its methylated derivatives: A shock-tube/TOF-MS and modeling study, J. Phys. Chem. A 123, 9893 (2019).
[3] L. Golka, D. Gratzfeld, I. Weber, M. Olzmann, Temperature- and pressure-dependent kinetics of the competing C–O bond fission reactions of dimethoxymethane, Phys. Chem. Chem. Phys. 22, 5523 (2020).
[4] A. Busch, N. González-García, G. Lendvay, M. Olzmann, Thermal decomposition of NCN: shock-tube study, quantum chemical calculations, and master equation modeling, J. Phys. Chem. A 119, 7838 (2015).