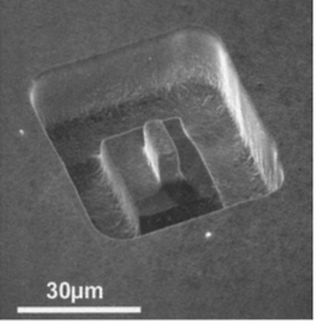
Electrochemical Microstructuring
We use ultrashort potential pulses with durations of typically 1 to 50 ns between a tool electrode and a workpiece to confine electrochemical reactions, e.g.electrochemical metal dissolution with submicrometer precision. This allows machining of metal surfaces similar to a conventional milling machine, however, on micrometer scale. [1] [2]
[1] R. Schuster*, V. Kirchner, P. Allongue, and G. Ertl, "Electrochemical micromachining”, Science 289, 98-101 (2000).
[2] X. Ma, A. Bán, and R. Schuster, “Electrochemical Machining of Gold Microstructures in LiCl/Dimethyl Sulfoxide”, ChemPhysChem 11, 616-621 (2010).