Thermodynamics and Kinetics of Electrochemical Processes
Electrochemical Microcalorimetry
With a specially designed microcalorimeter we measure the heat exchange upon electrochemical processes with tiny conversions down to a few percent of a monolayer of an electroactive surface species [1].
The reversible part of this heat corresponds to the reaction entropy of the overall process, including alsocharge neutral side processes The irreversible contribution reflects the overpotential of the reaction.
For that reason, electrochemical microcalorimetry is our method of choice to investigate thermodynamics as well as kinetics of Ec-surface processes.
Currently we investigate adsorption processes and DL charging, initial steps of metal deposition and electrocatalytical reactions.
Intial steps of metal deposition
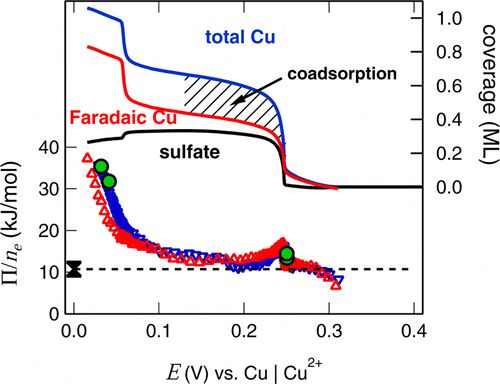
The electrodeposition of the first layers of ametal on a foreign substrate often starts at potentials positive to the equilibrium potential of the metal bulk deposition. This so-called underpotential deposition is usually accompanied by co-adsorption of anions from solution.We use standard electrochemical methods and EC microcalorimetry to disentangle these processes , e.g., for Cu and Ag UPD on Au(111) [2, 3]. From measurements of the heat flux we derive information on the reaction pathways, e.g., for Cu-bulk deposition or Ag-deposition form cyanide complexes.
Electrocatalytic reactions
We investigate electrocatalytic reactions, e.g., the hydrogen and oxygen evolution reactions on Pt with standard electrochemical methods and electrochemical microcalorimetry in order to identify reaction steps, side processes and reaction intermediates.
Adsorption processes and double layer charging
Upon polarization of an electrode in solution the composition of the electrode-electrolyte interface drastically changes, often involving adsorption of solution species on the electrode surface.
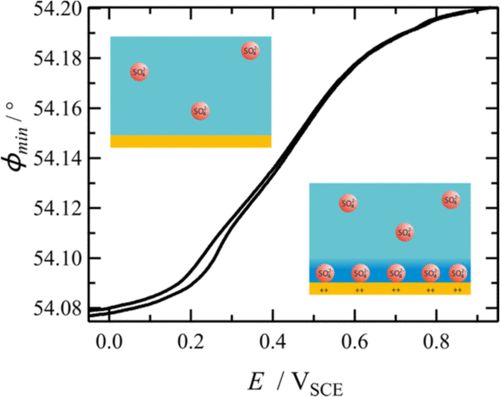
We investigate double layer charging and ionic or molecular adsorption by electrochemical microcalorimetry and surface plasmon resonance, for example sulfate [4], SDS [5] and pyridine adsorption on Au(111) , in order to derive information on the type and surface excesses of the adsorbed species and on accompanying processes like water restructuring on the electrode surface.
[1] S. Frittmann, V. Halka, C. Jaramillo, and R. Schuster, An improved sensor for electrochemical microcalorimetry, based on lithiumtantalate, Rev. Sci. Instr. 86, 064102 (2015).
[2] S. Frittmann and R. Schuster, Role of Anions During the Cu Underpotential Deposition on Au(111): A Microcalorimetric Investigation, J. Phys. Chem. C 120, 21522-21535 (2016).
[3] S. Frittmann, V. Halka, and R. Schuster Identification of Non-Faradaic Processes by Measurement of the Electrochemical Peltier Heat during the Silver Underpotential Deposition on AU (111), Angew. Chem. Int. Ed. 53, 4688 - 4691 (2016).
[4] K. Schlag, J. Braun, D. Nattland, and R. Schuster, Unraveling Compositional Changes of the Double Layer upon Sulfate Adsorption on Au(111) by Surface Plasmon Resonance, J. Phys. Chem. 123, 24598-24608 (2019).
[5] K. R. Bickel, A. E. Timm, D. Nattland, and R. Schuster, Microcalorimetic determination of the Entropy Change upon the Electrochemically Driven Surface Aggrefation of Dodecyl Sulfate, Langmuir 30, 9085-9090 (2014)