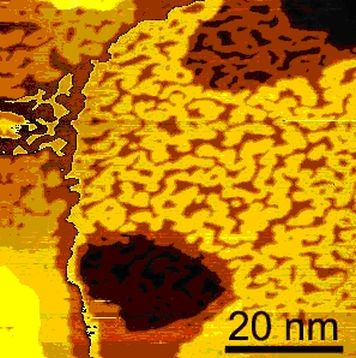
Ordering Processes on Surface
In electrochemical systems one can induce phase transitions of the surface system by shifting the electrochemical potential. If the potential pulse is directly applied to the tip of an electrochemical STM, such phase transitions can be driven very fast. [1]
We drove such phase transitions on a Au(111) surface within a few nanoseconds.
[1] R. Schuster*, D. Thron, M. Binetti, X. Xinghua and G. Ertl, "Two-dimensional nanoscale self-assembly in a gold surface by spinodal decomposition", Phys. Rev. Lett. 91, 066101 (2003).