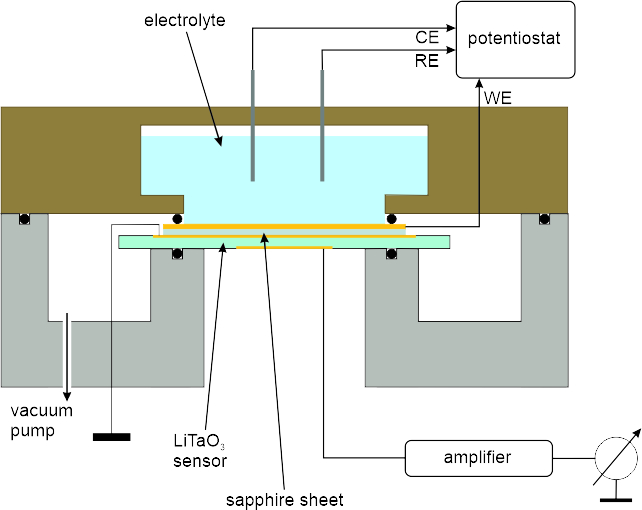
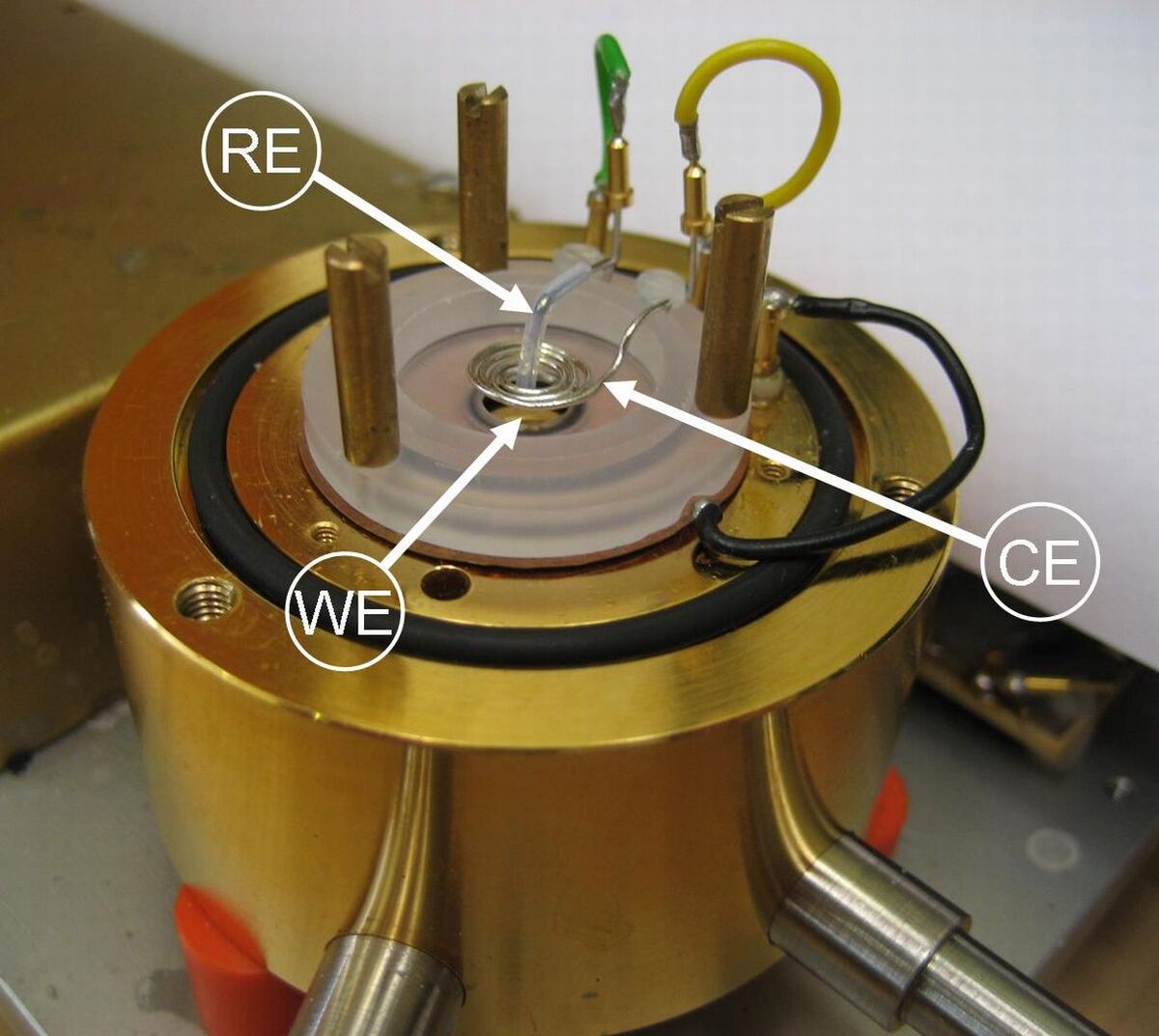
Electrochemical Microcalorimetry
-
The measurement of heat changes upon electrochemical reactions provides valuable information on the entropy of reaction as well as on possible irreversible reactions accompanying the charge transfer. We developed an experimental approach, which allows the investigation of these heat changes upon electrochemical reactions.
In electrochemical environment calorimetric measurements usually require large conversions, due to the high heat capacity of electrode and electrolyte. We achieved to measure submonolayer conversions with high sensitivity by adapting the calorimetric method, introduced by David King's and Charles Campbell's groups for the measurement of heats of adsorption in UHV [1], to electrochemical systems. Combining the use of a thin electrode-sensor assembly with pulsed electrochemical reactions resulted in sensitivities high enough for measuring heat changes upon conversions of a few percent of a monolayer (► fig. 1 and 2). Calibration of the calorimeter, e.g., with the Fe2+/Fe3+ redox reaction allows quantitative determination of the evolved heat and therefore of thermodynamic quantities of electrochemical reactions with submonolayer conversion.
[1] J. T. Stuckless, N. A. Frei and C. T. Campbell, Rev. Sci. Instrum. 69 (1998) 2427.
[2] S. Frittmann, V. Halka, C. Jaramillo, R. Schuster, Rev. Sci. Instrum. 86(6), 2015.